Study 5
Multicenter double-blind study examining the antidepressant effectiveness of the hypericum extract LI 160
Au: Hänsgen-K-D, Vesper-J and Ploch-M
SO: J Geriatr Psychiatry Neurol 1994; (supl1) PP 15-18
Introduction
Previous placebo-controlled, double-blind trials of extract LI 160 have had a duration of 4 weeks, in keeping with the drug trial guidelines for antidepressants. In this study, the trial drug was given for 6 weeks. To enable this, the so-called observation group design was chosen, in which the patients in the placebo group are given the trial medication for the last 2 weeks of the study.
Description
72 patients, aged 18-70 years, with major depression according to DSM-III-R from a total of 11 neurology and psychiatry practices or general practices were given Jarsin 300 (0.9 mg total hypericin) or a placebo for 6 weeks. In weeks 5 and 6, patients in both groups took the trial medication.
Further inclusion criteria were a total score on the Hamilton Depression Scale (HAMD) of 16 or more and a duration of their depressive episode from 2 weeks to a maximum of 6 months.
Patient compliance was evaluated by counting the tablets returned at each follow-up point. Study participants were informed that all patients would receive the trial medication for at least 2 weeks during the course of the study.
Results
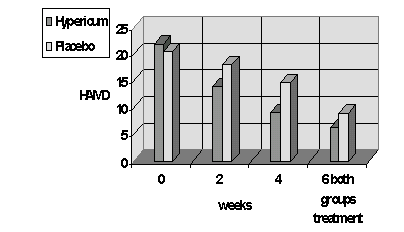
HAMD score

Depression scale (D-S)
BEB
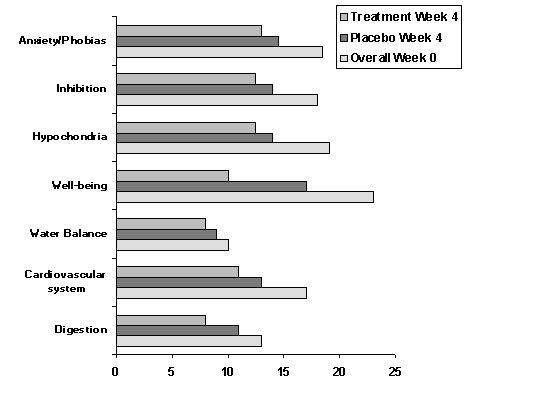
CGI
Side effects
One patient in the treatment group complained about sleep disturbance and two in the placebo group complained about gastrointestinal symptoms.
Researchers' comments
In interpreting the results, it must be borne in mind that the participants in the trial knew that they would receive the trial medication for at least 2 weeks. Most of the patients treated with the trial drug benefited from taking hypericum extract, as is shown by the continuous fall in depressive symptoms from the start of the study to the end of week 6 of treatment. Participants in the placebo group had no real improvement in symptoms up to the end of week 4.
Since the influence of certain doctors or practices are eliminated from the overall results because of the large number of practices supplying subjects, the results of a study such as this are particularly powerful. Because of its potent and specific efficacy with few or no side effects, hypericum extract LI 160 can be recommended as an antidepressant for treatment of depressed outpatients.
Copyright © 1996 by Harold H. Bloomfield, M.D. and Peter McWilliams
Disclaimer - Copyright - Contact
Online: buildfreedom.org | terrorcrat.com / terroristbureaucrat.com