D-S score
Study 1
Effectiveness and tolerance of the hypericum extract LI 160 in comparison with Imipramine: Randomized double-blind study with 135 outpatients
AU: Vorbach-EU; Hubner-WD; Arnoldt-KH
AD: Psychiatrische Klinik im Elisabethenstift, Darmstadt, Germany.
SO: J-Geriatr-Psychiatry-Neurol. 1994 Oct; 7 Suppl 1: S19-23
Description
135 patients, aged 18-75 years, 71 males and 64 females, were given indistinguishable tablets of either hypericum extract LI 160 0.9 mg (standardized hypericin content) x 3 or Imipramine 25 mg x 3 for six weeks in a randomized double-blind trial.
Inclusion criteria were typical depression according to DSM-III R with a single episode (296.2) or recurrent episodes (296.3), neurotic depression (300.4) and adjustment disorder with depressed mood (309.0).
Exclusion criteria were severe depression requiring inpatient treatment, schizophrenia or marked agitation requiring additional medication; a known history of attempted suicide or acute suicidal state, chronic alcohol and drug dependency and acute confusional state. Use of drugs with cerebral effects, especially psychotropic drugs, was not permitted. In addition, the patients must not have been taking MAO-inhibitors within the previous 2 weeks or drugs for research purposes within the previous 3 months.
There was a washout phase of at least 2 weeks before the start of the study. All non-psychotropic drugs were permitted and recorded on specific data sheets.
The patients had a thorough medical checkup with neurological status and routine Lab-parameters at the start and end of the study.
They were questioned specifically about possible side effects.
Target parameters were the Hamilton Depression Scale (HAMD), the von Zerssen Depression Self-rating Scale (D-S) and the Clinical Global Impressions (CGI).
Compliance was monitored by counting the tablets remaining at every visit. The classification of patients into different diagnostic groups is shown in Table 3.
Results
HAMD score
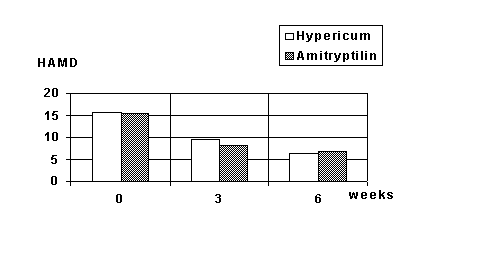
The mean HAMD fell from 20.2 to 8.8 in the hypericum group and from 19.4 to 10.7 in the Imipramine group. The reduction of HAMD score was significant with p< 0.001 in both groups. There were no statistically significant differences between the groups.
Figure 1 shows the HAMD-score at entry and after 2, 4 and 6 weeks.

D-S score
The mean D-S score fell from 39.6 to 27. 2 on hypericum and from 39.0 to 29.2 on Imipramine.
CGI score
The CGI score for therapeutic effect rose from 1.3 to 3.1 in the hypericum group and from 1.2 to 2.7 in the Imipramine group.
· The CGI score for change in status was slightly more positive in the hypericum group than in the Imipramine group (see Figure 5); 41% were greatly improved, 35% much improved, 12% slightly better and 12% experienced no change. None of the hypericum patients experienced worsening of their condition. The comparative results for Imipramine were 34% greatly improved, 27% much improved, 17% slightly better, 17% unchanged, 3% somewhat worse and 2% much worse.
The CGI score on change of illness severity also showed a trend towards better results with hypericum. 81.8% were classified as having improved on hypericum while 62.5% had improved on Imipramine. 18.2% were unchanged or the same compared to 34.4% in the Imipramine group. None of the hypericum patients and two of the Imipramine patients experienced worsening of their condition.
Effect on severe depressions
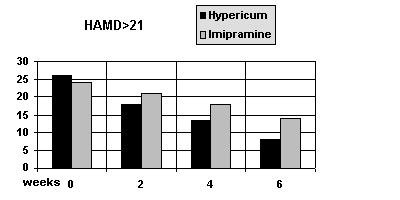 In the subgroup with patients with a HAMD> 21 (severe depressions) there was a statistically significant trend towards superiority of hypericum (p<0.05) The mean score fell from 25 to 9 in the hypericum group and from 24 to 14 in the Imipramine group (see Figure 2).
In the subgroup with patients with a HAMD> 21 (severe depressions) there was a statistically significant trend towards superiority of hypericum (p<0.05) The mean score fell from 25 to 9 in the hypericum group and from 24 to 14 in the Imipramine group (see Figure 2).
ADRs
Adverse drug effects (ADRs) occurred in 8 patients on hypericum (11.9%). The most frequent symptoms were dry mouth (4 cases) and dizziness (2 cases). 11 patients mentioned ADRs on Imipramine. The most frequent were dry mouth (9 cases), dizziness and anxiety (3 each) and constipation (2 cases). 10 of 11 symptoms were said to be mild with hypericum and 15 were classified as mild, 4 as moderate and 3 as severe on Imipamine (see Table 4).
There were no changes in laboratory parameters or clinical status.
The researchers' comments
The clinical efficacy of Imipramine has been demonstrated in more than 1000 therapeutic studies.
The normal dose of Imipramine for outpatients is 50-150 mg/day. This study used a dose of 75 mg because
1. It is generally agreed upon that 50 mg is a sufficient dose for outpatient treatment, especially with older subjects, who often require lower doses because of slower elimination.
2. With a higher dose there would be a risk that it would be obvious which patients were receiving which drug because of the typical side-effects on Imipramine. This would interfere with the double-blind structure.
This study did show Imipramine to have similar effects and side-effects as in previous studies.
Hypericum was clearly superior to Imipramine in terms of patient tolerance and fewer side effects.
The dose of hypericum was high in this study (2.7 mg hypericin daily). The authors conclude that more studies are needed to determine the dose-response effect with hypericum in order to find the optimum dose of treatment for different conditions.
The finding of superior effect on severe depressions with treatment of hypericum needs to be complemented with more studies on severe depressions and also with comparative studies using higher doses of Imipramine. The Drug Monitoring study by Woelk et al. on 3,250 patients did not show better effect on severe depressions, but that study had a very high proportion of women. In the criteria-based review by Ernst this study scored highest in terms of quality (74 points out of 110).
Copyright © 1996 by Harold H. Bloomfield, M.D. and Peter McWilliams
Disclaimer - Copyright - Contact
Online: buildfreedom.org | terrorcrat.com / terroristbureaucrat.com