Drug Monitoring Studies
Summary
Drug monitoring studies are made in order to collect knowledge on licensed drugs on a large scale. Drug monitoring studies usually allow inclusion of a much greater number of patients than controlled clinical studies and are thus primarily of use in establishing side effects, but may also predict efficacy, especially in different age groups or with different severity of illness.
As far as we know, there have been two drug-monitoring studies performed so far: one on 3,250 patients taking the high-dose preparation Jarsin 300 and one on 1,040 patients taking the low-dose preparation Jarsin.
Results are summarized in Table 4.
Study 10
Benefits and risks of the hypericum extract LI 160: Drug-monitoring study with 3,250 patients
AU: Woelk-H; Burkard-G; Grunwald-J
AD: Psychiatrisches Landeskrankenhaus und Akademisches Lehrkrankenhaus, Universitat Giessen, Germany.
SO: J-Geriatr-Psychiatry-Neurol. 1994 Oct; 7 Suppl 1: S34-8
Description
Effectiveness and acceptance of a 4-week treatment with hypericum extract LI 160 Jarsin 300 (=3x 0.9 mg total hypericin) were investigated by 663 private practitioners. The results of the 3,250 patients (76% women and 24% men), were recorded using data sheets. Eight typical symptoms were recorded (see Figures 12 and 13). Severity of depression was measured with von Zerssen's D-S self-rating schedule. The severity and duration of all possible side effects were also recorded. Finally the doctor and patients evaluated the course of treatment together, rating the patient's condition as either worse, unchanged, better, symptom-free (see Figure 10). The different specialties of the physicians involved are summarized in Table 5.
Results
General improvements
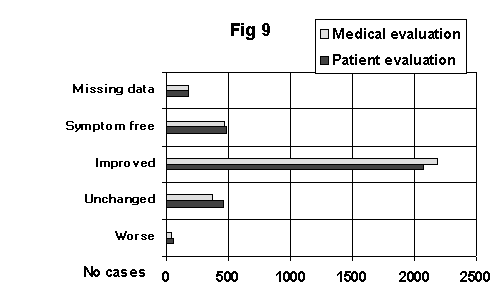
The proportion of improvement in depressive and somatic symptoms was similar to other studies on hypericum. About 80% of the patients felt better and 15% unchanged or worse when asked to make an overall judgment. Figure 9 shows these results.
The D-S scale
All side effects were mild and there were no significant differences between the two groups.When measured with the D-S scale, the response rate was between 60% and 70%. Figures 10 and 11 show the results on the D-S scale according to severity of symptoms and different age groups.
Severity and frequency of symptoms
All side effects were mild and there were no significant differences between the two groups.It was found that the majority of patients got better, but only a small proportion achieved complete freedom from symptoms. Figure 12 shows the frequencies in percent of the symptoms mentioned. Figure 13 shows the corresponding severity of symptoms.
Side effects
Side effects were rare (seen in only 2.4% of the subjects) and mild. Table 6 shows the most common spontaneously mentioned side effects.
Dropouts
The dropout frequency was 1.45%. Table 7 shows the reasons for dropouts in this study.
Researchers' comments
All side effects were mild and there were no significant differences between the two groups.For evaluation of side effects, it is important to mention that about 50% of the patients already had "gastrointestinal symptoms" before the study. Anxiety, fatigue and dizziness are also frequently associated with depression. It is therefore very hard to scientifically conclude whether these "side effects" were due to depression or were because of the treatment with hypericum.
The side-effect rate was only 2.4%, compared to 19% in a similar study on Fluoxethine (Prozac).
The dropout rate was about five times lower than in similar studies performed on "the new generation" synthetic antidepressants.
Our comments
Our clinical experience indicates that side effects on synthetic antidepressants are much more prevalent than on hypericum.
Our experience is that loss of sexual interest, orgasm and impotence problems are far more frequent, but are underreported in many clinical studies for the following reasons:
Copyright © 1996 by Harold H. Bloomfield, M.D. and Peter McWilliams
Disclaimer - Copyright - Contact
Online: buildfreedom.org | terrorcrat.com / terroristbureaucrat.com